BOUTIQUEResources
MES, the abbreviation for
2-(N-morpholino)ethanesulfonic acid, is a synthetic zwitterionic buffer. It was
developed in 1966 by Norman Good and his team, belonging to the famous
"Good's Buffers" family, and is one of the commonly used and
important pH buffers in biochemical and molecular biology experiments.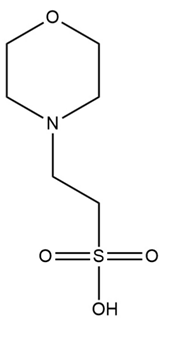
As a member of the "Good's Buffers" family, MES possesses a series of superior characteristics:
① Ideal pKa value: The pKa of MES is 6.15 at 25°C. According to buffer theory, a buffer is most effective within ±1 pH unit of its pKa. Therefore, the ideal buffering range for MES is pH 5.5 - 6.7. This range covers the critical pH for many biochemical reactions.
②Good biocompatibility: It causes minimal interference with enzyme activity and cell metabolism; has low cytotoxicity, making it commonly used in cell culture and protein studies.
③Chemical inertness: Does not participate in or interfere with most biochemical reactions; does not strongly chelate essential divalent cations (such as Mg2?, Ca2?), which is crucial for the activity of many enzymes (e.g., ATP-related enzymes).
④High water solubility: Highly soluble in water, allowing convenient preparation of high-concentration stock solutions (e.g., 1M).
⑤Low UV absorption: Has very low absorbance at wavelengths above 260 nm, minimizing interference with UV spectrophotometric detection of nucleic acids or proteins.
⑥Good thermal stability: Relatively stable at high temperatures.
Given its superior physicochemical properties, MES offers significant advantages compared to other commonly used buffer components.
MES vs. PBS (Phosphate Buffered Saline): PBS has strong buffering capacity in the pH 7.0-7.4 range but can chelate calcium and magnesium ions and may inhibit certain enzyme activities. MES does not have these issues and can work in more acidic pH conditions.
MES vs. Tris: Tris has a pKa of 8.07 and is a classic buffer for the alkaline pH range. MES serves as a complement in the acidic to near-neutral range. Tris is temperature-sensitive and may participate as an active group in certain reactions.
MES vs. HEPES: HEPES has a pKa of 7.55 and is one of the commonly used neutral buffers in cell culture. MES can serve as a complement to HEPES in more acidic pH ranges.
Integrating the above advantages, MES finds wide application across various fields of life science research:
? Protein Electrophoresis and Purification: SDS-PAGE / Chromatography.
? Cell Biology: Used to prepare culture media or perfusion fluids to maintain pH stability in the extracellular environment.
? Organelle Isolation: Provides a buffered environment in homogenization and centrifugation media for subcellular fractionation.
? Biochemistry and Enzymology: Provides a stable pH environment for enzyme activity assays and enzyme kinetic studies.
? Molecular Biology: Serves as a buffer component in Northern Blot and certain nucleic acid hybridization and detection experiments.
In summary, MES is a high-performance, widely applicable buffer. Its core value lies in providing a stable, inert, and broadly compatible chemical environment within the weakly acidic physiological pH range of 5.5 - 6.7, ensuring the smooth progress of life science experiments.
As a long-term, fixed supplier for many globally renowned culture media manufacturers and pharmaceutical companies, PanReac AppliChem provides reliable MES for researchers and pharmaceutical enterprises, leveraging its exceptional product quality and stringent quality control.
|
Product Code |
Chinese Name |
English Name |
CAS |
|
A0689 |
2-嗎啉乙磺酸 |
MES anhydrous BioChemica |
4432-31-9 |

XMJ is the exclusive general distributor of PanReac AppliChem in China, providing users with comprehensive technical support and after-sales service. If you are interested in the products, please feel free to:
· Call XMJ’s customer service hotline: 400-050-4006
· Visit the official website: rmb365.cn to learn more information.


.png) 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號